Product Description
GMP grade Protein-Free Cryopreservation Medium utilizes a combination of osmotic protectants, non-penetrating protectants, cell sedimentation stabilizers, and cell membrane protectants to provide comprehensive low-temperature protection for cells. This medium lowers the freezing point during cryopreservation, enhances cell membrane permeability to water, allows cellular water to exit the cells before freezing, and prevents or reduces ice crystal damage to cells. It also reduces the concentration of electrolytes around the cells during freezing to prevent electrolyte damage and delays the compression of cells against each other, thus effectively improving cell recovery rates and viability.
Traditional cell freezing media consist of a mixture of culture medium, serum, and DMSO in specified proportions. Due to the complex nature of the serum component and significant batch-to-batch variability, its use is limited and typically requires programmed freezing, which is time-consuming and labor-intensive. GMP grade Protein-Free Cryopreservation Medium is a serum-free, animal origin-free, protein-free, chemically defined solution that does not require programmed freezing. Compared to traditional cell freezing media, it offers significant advantages and also shows a noticeable improvement in cell viability post-thaw.
GMP grade Protein-Free Cryopreservation Medium is recommended for the cryopreservation of various conventional mammalian cells, especially suitable for serum-free cultured stem cells, immune cells, and protein expression cells.
Table 1: Comparison of the characteristics of conventional cell cryopreservation medium and Meiluncell GMP Grade Protein-Free Cryopreservation Medium
| Comparison Items | Conventional Cryopreservation Medium | Meiluncell GMP Grade Protein-Free Cryopreservation Medium |
| Serum | Yes | No |
| Protein | Yes | No |
| Ingredients Defined | No | Yes |
| Programmed Cooling Process | Required | Not required |
| Solution Preparation | Preparation when used | Ready-to-use, no preparation required, stored at 4°C |
| Endotoxin | >0.5EU/mL | ≤0.5EU/mL |
| Survival Rate of Resuscitation | Normal (low cell survival rate when microfrozen) | High (high cell survival even when microfrozen) |
| Batch Difference | High batch variation | Minimal batch variation |
| Whole Plate Freezing | Not feasible | Possible and convenient |
| Safety | Risk of contamination with viruses, molds and mycoplasmas of animal origin | No risk of contamination with animal-derived viruses, molds, mycoplasmas, etc. |
| Application for Pharmaceutical Excipients | Extremely difficult | Relatively easy |
| Can be used in the clinic? | No, contains animal serum, protein | Yes, chemically defined, no animal source ingredients, no protein |
Quality Control System
Meiluncell Grade Protein-Free Cryopreservation Medium, production and R & D quality control in strict accordance with GMP standards, the product has obtained the mMedical Device Filing (record number 苏苏械备20210235), and carried out the mouse systemic acute toxicity experiments, rabbit hemolytic experiments, and other clinical toxicology studies, indicating that the product is safe and reliable. In the process of CDE registration of pharmaceutical excipients, we can provide complete process, quality, toxicology research data, in line with clinical regulatory requirements, chemical composition, endotoxin levels lower than the national standard for injections, which can maximize the speed of the customer’s clinical trials. We welcome joint filing.
Product Advantage
- Safety: Serum-free, free from animal-derived components, protein-free, and chemically defined.
- Efficiency: Effectively improves cell recovery and survival rates, with most cells (including mesenchymal stem cells) achieving recovery rates of over 90%.
- Stability: Does not affect stem cell phenotype or growth state, and maintains the pluripotency of stem cells.
- Convenience: Ready-to-use product that does not require programmed freezing; can be directly stored at -80°C overnight before long-term storage in liquid nitrogen.
- Quality: Strict quality control ensures product performance comparable to imported products; raw materials are either self-produced or selected from strictly tested and purified low-endotoxin, high-purity pharmaceutical-grade sources; prepared with Water for Injection, manufactured in compliance with GMP standards, ensuring stable processes and minimal batch-to-batch variability.
- Reliability: Already used for cryopreservation of various cell types including stem cells, immune cells, tumor cells, and transformed cell lines.
Production process: This product is prepared by using Water-For-Injection (WFI), and the production procedure is strictly in accordance with the cGMP management regulations, and is completed in the clean room for the record of in vitro diagnostic reagents. The company has obtained ISO9001 quality system certification.
Product packaging form: 100mL/bottle
Performance
1. Cell recovery survival rate
The Meiluncell® GMP Grade Protein-Free Cryopreservation Medium group is comparable to the International C brand serum-free cryopreservation medium group in terms of survival on most cells, and in some cases the Meiluncell® group is even more viable (Figures 1&2).
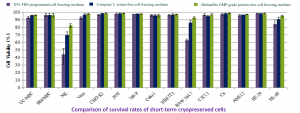
Figure 1: Comparison of survival rates of multiple cells after resuscitation with Meiluncell® GMP Grade Protein-Free Cryopreservation Medium, imported C brand serum-free cryopreservation medium, and programmed cryopreservation medium (20%FBS+10%DMSO) frozen at -80°C for 3-17 days.
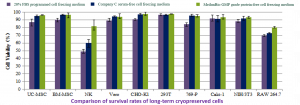
Figure 2: Comparison of survival rates of multiple cells after resuscitation with Meiluncell® GMP Grade Protein-Free Cryopreservation Medium, international C brand serum-free cryopreservation medium, and programmed cryopreservation medium (20%FBS+10%DMSO) frozen at -80°C for 3 months.
2. Comparison of the Adhesion of MSCs after resuscitation
After cell resuscitation, the cell number was adjusted to 1×10^5/ml, UC-MSC were cultured with serum-free medium, and BM-MSC were cultured with DMEM-L+10% FBS, and the culture medium changed and observed the cell adhesion situation after 24 h.
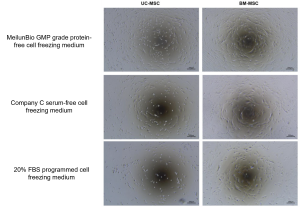
Figure 3: Comparison of cell adhesion after resuscitation of MSCs frozen with Meiluncell® GMP Grade Protein-Free Cryopreservation Medium, international C brand serum-free cryopreservation medium, and programmed cryopreservation medium (20% FBS+10% DMSO).
3. Cell growth after resuscitation
Cells were cultured for 48h after resuscitation and growth was observed. Meiluncell® GMP Grade Protein-Free Cryopreservation Medium group showed comparable or even better cell status compared with the international C brand serum-free cryopreservation medium (Fig. 4), and the growth of MSCs and other wall-adherent cells would not be affected after freezing.
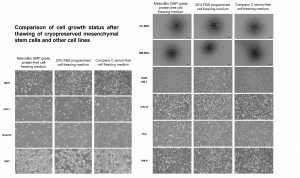
Figure 4: Comparison of cell growth status after resuscitation with Meiluncell® GMP Grade Protein-Free Cryopreservation Medium, international C brand serum-free cryopreservation medium, and programmed cryopreservation medium (20% FBS+10% DMSO) for MSCs and other cell lines.
4. Effects on the expression of mesenchymal stem cell markers
Cell marker expression was assessed by flow cytometry after the cells were resuscitated and passaged twice. Meiluncell® GMP Grade Protein-Free Cryopreservation Medium group was able to maintain positive expression of CD105 and CD90, and negative for CD45, with no differences observed in marker expression from the programmed cryopreservation medium (Figure 5).
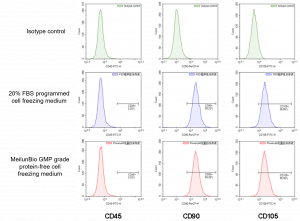
Figure 5: Expression of cellular markers after resuscitation of human umbilical cord MSCs cryopreserved with Meiluncell® GMP Grade Protein-Free Cryopreservation Medium, programmed cryopreservation medium (20% FBS+10% DMSO)
Identification of the differentiation potential of cryopreserved human umbilical cord mesenchymal stem cells.
Cells cryopreserved in Meiluncell® PWL099 retained trilineage differentiation potential, with adipogenic differentiated cells forming visible lipid vacuoles, showing typical adipogenic differentiation; osteogenic differentiated cells forming a large number of calcaneal nodules, showing typical osteogenic differentiation; and chondrocyte spheroids sectioned and stained demonstrated the presence of proteins such as chondroitin sulphate and gliadin sulphate, showing typical osteogenic chondrogenic differentiation (Figure 6).
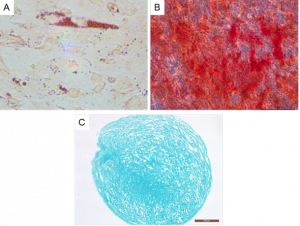
Figure 6: The recovered MSCs were differentiated into (A) adipocytes, (B) osteocytes and (C) chondrocytes, and were stained with (A) Oil Red O, (B) Alizarin Red and (C) Alcian Blue respectively.
Shipping and Storage
- Storage:Store at 2-8°C, 12 months
- Shipment:
Usage Statement
Research Use Only (RUO)
All sales are subject to the General Terms and Conditions of Sale set forth on our website.